On Thursday, April 23, we hosted our 9th virtual briefing of the Private Sector Roundtable (PSRT) for global health security. We were joined by Rear Admiral Nancy Knight, MD, Deputy Incident Manager, Global, CDC COVID-19 Response, to learn more about how the CDC is supporting Africa in responding to COVID-19.
Dr. Knight discussed Africa’s growing pandemic, which is in the “acceleration phase.” While most new COVID-19 cases are coming from Europe and the Americas (each of these regions account for 44 percent of new cases in the last 24 hours), the number of cases in Africa is expected to increase rapidly. At present, official estimates are reporting about 16,000 cases and 720 deaths, likely low compared to actual figures. She noted Imperial College’s modeling shows that Africa could see more than one billion infections and two million deaths due to COVID-19 – which may be an underestimate given assumptions of health system capacity and ability to implement social distancing measures.
The CDC is examining key gaps within African countries’ health systems that are specific to COVID-19, including those related to lab systems, emergency response and pandemic preparedness planning, infection prevention and control, surveillance and vaccine capabilities. Some of the major gaps are related to hygiene due to lack of access to clean water, soap and alcohol-based hand sanitizer. In response, the CDC is providing recommendations to communities about keeping water as clean as possible and alternatives to handwashing. Similarly, the CDC is providing operational guidance to reduce strain on health care settings, such as triaging patients, performing safe and dignified burials and ensuring continuity of health care services for those with chronic disease – applying lessons learned from PEPFAR and its approach to multi-month dispensing of HIV medicines.
Dr. Knight also described how the CDC’s incident management team is supporting countries’ health leaders on preparedness and response as well as serving as technical advisors to the Africa CDC to help develop strategy and guidance documents, event-based surveillance systems and modeling. She noted how Uganda is building on the extensive work that has been supported through the Global Health Security Agenda, with the support of the PSRT, including establishing the first-ever border health program and helping staff trained at Entebbe Hospital for Ebola to pivot and now respond to COVID-19.
In terms of opportunities for private sector engagement, Dr. Knight highlighted the following areas: build and scale testing capacity, especially outside of urban areas; secure personal protective equipment and other commodities; innovate quickly to ramp up intensive care capabilities; and develop risk communications.
Finally, Dr. Knight underscored the “secondary implications” of COVID-19 and the importance of making sure that people living with chronic disease stay healthy and remain home. She emphasized the need for health systems to continue to address vaccine preventable diseases and ensure ongoing access to immunizations.
Pandemic Spread
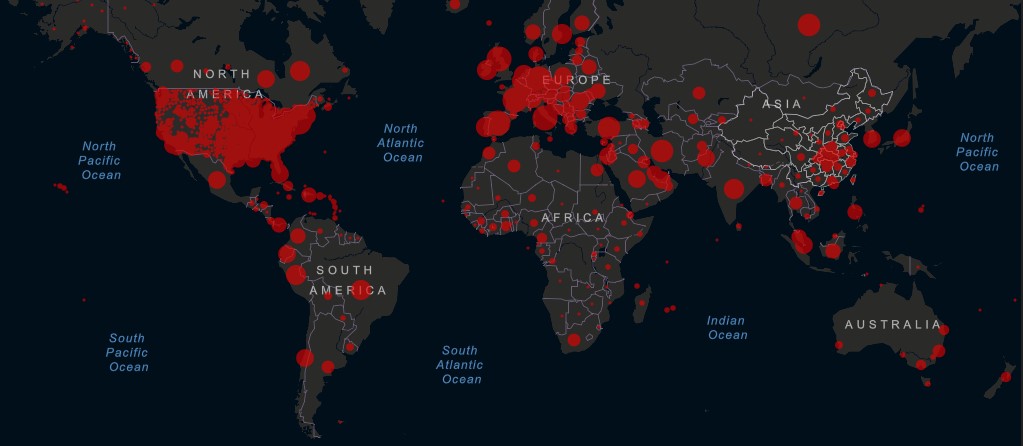
Some countries, which previously appeared to have their epidemics under control (such as Singapore), are seeing a spike in cases. In many more countries, infections continue to grow along an exponential curve, despite social distancing.
In the U.S., the global epicenter of the pandemic, some states are discussing lifting distancing restrictions and reopening their economies, even as cumulative deaths rose to 46,851 this week. Experts note that U.S. testing capacity needs to increase at least three-fold.
Worldwide, as of Thursday, April 23 at 10:30am ET, the Center for Systems Science and Engineering at Johns Hopkins University reported 2,658,387 confirmed cases and 185,434 deaths attributed to COVID-19. With 843,937 reported cases of COVID-19, the U.S. accounts for nearly a third of the global burden.
“While locking down our economy is crucial for saving lives now, it has tremendous consequences for the poorest among us—as people of color and low-income Americans are disproportionately losing livelihoods, and lives. In the face of an ineffective nationally coordinated response, insufficient data, and inadequate amounts of protective gear and testing, we need an exit plan.”
Rajiv Shah, President, Rockefeller Foundation
Industry Developments
On Friday, April 17, the National Institutes of Health announced a coalition, Accelerating COVID-19 Therapeutic Interventions and Vaccines, to develop an international strategy for a coordinated research response. Members include the European Medicines Agency, the U.S. Department of Health and Human Services, U.S. Centers for Disease Control and Prevention, U.S. Food and Drug Administration and more than a dozen biopharmaceutical companies, including AbbVie, Johnson & Johnson, MSD, Novartis and Pfizer.
Notable progress – and setbacks – are being made across efforts to combat COVID-19:
- In an effort to produce high-quality data on hydroxychloroquine’s efficacy in treating COVID-19, on Monday, April 20, Novartis announced it would begin a large scale, double-blind, randomly controlled trial. Recruitment for the Phase III trial will begin in the next few weeks. This trial would add to the limited body of data that currently suggests mixed results. A retrospective study of 368 patients treated by the U.S. Veterans Health Administration saw an overall increase in mortality among patients treated with hydroxychloroquine alone. Those patients who received hydroxychloroquine plus azithromycin fared better, but the combination showed no positive impact on the need for ventilation.
- On Tuesday, April 21, the FDA issued an emergency use authorization for LabCorp’s at-home COVID-19 testing kit. The kit – the first of its kind to be approved in the U.S. – will be available first to health care workers and then the general public in the coming weeks.
- Implementing widespread testing remains a challenge in the U.S. as the highly anticipated Abbott Labs rapid test has produced inconsistent results. Researchers at the Cleveland Clinic revealed the test produced an almost 15 percent false-negative result in a sample of 239 specimens.
- Researchers at the Jenner Institute and Oxford Vaccine Group initiated trials of their recombinant viral vector vaccine candidate this week in 1,112 healthy young adults. Anticipating that the vaccine will prove effective, the U.K. government is preparing large-scale production capacity, aiming to produce a million doses by September.
- Germany’s first vaccine trial will begin in the next few weeks following BioNTech’s announcement on Wednesday, April 22 that the company has received approval to start Phase I/II clinical trials of four vaccine candidates. BioNTech and Pfizer are collaborating to develop the mRNA vaccine candidates and expect to receive approval to begin trials in the U.S. shortly.
In light of such rapidly progressing developments, on-going calls to “facilitate the equitable and affordable access” to innovative diagnostics, medicines and vaccines take on new urgency. Some companies are answering this call: In announcing the Phase III trial of hydroxychloroquine, Novartis promised to make the drug’s intellectual property available broadly if the medicine is approved to treat COVID-19.
Comings and Goings
Effective Monday, April 20, Sir Andrew Witty, President of UnitedHealth Group and former CEO of GlaxoSmithKline, will co-lead the WHO’s efforts to accelerate development of a COVID-19 vaccine. He will take a leave of absence from his current position.
On Tuesday, April 21, the U.S. Department of Health and Human Services announced it has replaced Rick Bright as director of the Biomedical Advanced Research and Development Authority (BARDA), the agency overseeing COVID-19 vaccine and treatment development. The sudden removal is seen by some as a sign that the Trump administration grew frustrated with Bright’s refusal to promote blanket use of hydroxychloroquine. Bright has been reassigned to the National Institutes of Health. Gary Disbrow, Bright’s former deputy, will serve as acting director of BARDA.
From the Experts
“There’s a pandemic every three years. That’s not the black swan. The black swan is the lack of coordination between governments to deal with it.”
Paul Polman, former CEO, Unilever
Friday, April 17
“We have to give credit to Africa for getting ahead, I think it is very important to value the work that African countries have done so far.”
Dr. Silvia Lutucuta, Minister of Health, Angola
Friday, April 17
“It’s important that organizations involved in [data analysis] commit to doing it in a way that protects people’s information and that any data collected is used solely for responding to public health emergencies and for other crisis response efforts. Fighting the pandemic has required taking unprecedented measures across society, but it shouldn’t mean sacrificing our privacy.”
Mark Zuckerberg, Founder and CEO, Facebook
Monday, April 20
“This pandemic, and now the attack on WHO, is going to set countries back decades.”
Richard Horton, Editor, The Lancet
Monday, April 20
“We must not, especially now, let down our guard on immunizations. Access to vaccines for all has transformed our societies, but it is a public good that must be maintained to be effective, even in difficult times. Our overstretched health systems cannot bear any outbreaks of vaccine-preventable diseases.”
Dr. Hans Henri P. Kluge, WHO Regional Director for Europe
Monday, April 20
“There’s a possibility that the assault of the virus on our nation next winter will actually be even more difficult than the one we just went through.”
Robert Redfield, Director, CDC
Tuesday, April 21
Additional Resources
Reports from International Governments and Bodies
- WHO COVID-19 Information and Guidance
- WHO Situation Reports, April 20, April 21, April 22
- White House Coronavirus Task Force Press Briefings, April 20, April 21, April 22
- CDC Coronavirus Resource Page
- COVID-19 Health Systems Response Monitor
- NCD Alliance COVID resources relevant to NCDs
Funding and Policy Trackers
- International Monetary Fund Policy Tracker
- Kaiser Family Foundation Coronavirus Policy Tracker
- U.S. Chamber of Commerce Foundation Corporate Aid Tracker
- Devex Interactive Funding Tracker
Resource Pages and Market Research Literature
- JAMA Resource Center
- The Lancet COVID-19 Resource Centre
- PharmaIntelligence: Coronavirus – What will the Impact Be?
- Health Affairs Resource Center
- STAT Preparedness Tool
- International Association of National Public Health Institutes COVID-19 Resources
- U.S. Global Leadership Coalition COVID-19 Issue Briefs
Communications Toolkits
What We’re Reading
Meet the Top American Fighting COVID-19 at WHO, Reid Wilson, TheHill
The Health 202: Twelve Takeaways from the 1918 Flu Epidemic that Help us think about the novel coronavirus, Paige Winfield Cunningham, Washington Post
The Pandemic’s Hidden Victims: Sick or Dying, but Not From the Virus, Denise Grady, The New York Times
The Secret to Germany’s COVID-19 Success: Angela Merkel Is a Scientist, Saskia Miller, The Atlantic
The Remaking of Big Pharma in a Post-Pandemic World, Ethan Guillén and Melissa Chan, Foreign Policy
British Scientist to Head UN Task Force Distributing COVID-19 Vaccine as US Blocks G20 Agreement, Sarah Newey and Paul Nuki, The Telegraph
Politics May Kill Us, Not the Coronavirus, Eduardo J. Gómez and Sandro Galea, Think Global Health