As the Secretariat for the Private Sector Roundtable (PSRT) for global health security – a cross-industry coalition that supports countries in preparing and responding to health emergencies – we have been hosting a weekly series of insider discussions for companies with high-level global officials. Recent calls have featured executives from the U.S. Department of State, Asian Development Bank and the World Bank.
The most recent call (March 26) featured the important contributions of the World Bank to the COVID-19 response. Mukesh Chawla, Chief Adviser, Health Security and Rocio Schmunis, Senior Health Specialist, Health, Nutrition & Population spoke to PSRT members and invited guests about the World Bank’s recently approved $14 billion package of fast-track financing to assist countries in their efforts to prevent, detect and respond to the rapid spread of COVID-19 globally. They described how the Bank is putting unprecedented procedures in place to move forward quickly to enable countries to procure essential commodities to treat patients and protect health providers – noting that demand is outstripping supply. They added that the President of the World Bank Group stated on Monday, March 23, that it could deploy as much as $150 billion over the next 15 months.
The PSRT is facilitating connections between companies that have stock or production capability with the World Bank and its procurement arm, which maintains a running list of countries’ needs. If you are interested in learning more about joining the PSRT, please contact [email protected].
Global Funding Developments
The funding needed for the global COVID-19 response is exponentially greater than previous global crises. Costs associated with accelerating research and development, strengthening health systems and supply chains, supporting economic redevelopment and beyond are estimated to reach trillions of dollars. The Coalition for Epidemic Preparedness Innovations (CEPI) estimates $2 billion will be needed to develop a vaccine alone. The UN Economic Commission for Africa reports Africa may need $10.6 billion to cover increases in public health and healthcare spending. Reflections on China’s decisive action to restrict mobility show the response, while successful, came at great economic cost. As nations around the world introduce more stringent lockdowns, the economic impact is becoming more apparent. New estimates suggest U.S. unemployment could jump to 30%. African nations – with less than 1% of global cases, as calculated by WHO March 25 situation report – have already lost an estimated $29 billion. Already suffering losses to their tourism and trade industry, Latin American and Caribbean countries are reconciling the need for extensive social distancing measures with long term social and economic impacts.
Wealthier nations have begun offering citizens direct income replacement. The Danish government will pay 75% to 90% of employees’ salaries if businesses do not lay-off workers, and the Netherlands is providing up to 90% of wages for companies shuttered by COVID-19. The U.S. Senate passed a $2.2 trillion stimulus package early on Thursday, March 26, which the House of Representatives will now take up. Many argue that even this large a measure will not be enough to support the millions of Americans who are losing their jobs and health insurance. Lowerincome nations are unable to offer such economic stimulus. On Wednesday, March 25, the World Bank Group and International Monetary Fund issued a call to suspend debt payments from poor counties to preserve the liquidity needed to respond to the pandemic.
In the U.S., the end of last week saw private philanthropists and foundations contribute millions of dollars. On Thursday, March 19, the Rockefeller Foundation committed $20 million to accelerate short- and long-term pandemic preparedness and response and to address systemic gaps in basic human services. On Friday, March 20, a group of 18 foundations, companies and individuals launched the NYC COVID-19 Response & Impact Fund to support New York City nonprofits that provide a range of social services as the need for food, housing and employment becomes more acute. In Seattle, Washington, where the first cases of COVID-19 were reported in the U.S., a community fund supported by foundations such as the Bill & Melinda Gates Foundation, businesses including Target and Nordstrom and individual donors has collected $14.3 million. The first slate of grantees will be announced on Monday, March 30.
Notable Industry Developments
Researchers across the biopharmaceutical industry, academe, philanthropy and government are working in partnership to find viable medical countermeasures for the crisis. On Friday, March 20, WHO launched the multi-country SOLIDARITY Trial examining the four most promising COVID-19 treatments, among approved medications, including remdesivir, chloroquine and hydroxychloroquine, ritonavir/lopinavir and ritonavir/lopinavir with interferon-beta. This morning, Novartis announced that the company would lead a consortium of life science companies to begin pooling their expertise and resources to accelerate development of vaccines, diagnostics and treatments for COVID-19 in partnership with the Bill & Melinda Gates Foundation. Some these collaborations are revisiting existing compounds – testing everything from antiretrovirals for HIV to medicines for lupus – to find therapeutics to ease patient symptoms. Others are taking a longer-term view, developing new vaccine candidates to help populations achieve herd immunity and disrupt transmission. See below for more examples. A list of trials may be found here.
Treatment
- Existing antivirals – Anecdotal evidence early in the pandemic suggested remdesivir, Gilead Science’s compound originally designed to fight Ebola, could be effective in managing symptoms for patients with severe COVID-19. The antiviral
targeting RNA polymerase, a key enzyme for replication, is being studied in 75 sites globally, and results could be seen as early as April. - Existing arthritis drugs – Monday, March 23, the FDA greenlighted a Phase III randomized-control trial of Genentech rheumatoid arthritis therapeutic Actemra in collaboration with the Biomedical Advanced Research and Development Authority (BARDA). Actemra is an interleukin-6 receptor antagonist that is believed to dampen COVID-19 symptoms by preventing cytokine release syndrome, an antagonistic inflammatory response. Results are expected in May.
- Convalescent plasma – Takeda Pharmaceuticals is turning to a proven strategy of using purified blood plasma from recovered patients to boost the immune response
of new patients. Takeda foresees a speedy approval for its experimental treatment as the therapy uses the same manufacturing process as Takeda’s other approved immunoglobulin products. - Artificial antibodies – Regeneron Pharmaceuticals has isolated hundreds of antibodies with potential to neutralize the virus. Regeneron plans to select two lead candidates to include in a cocktail therapy and enter human trials by early summer.
Prevention
- Hydroxychloroquine finds another potential usage for post-exposure prophylaxis. Clinical trials in New York City and Minnesota are beginning to examine the efficacy to prevent emergence of symptoms in healthcare workers and others with confirmed exposures.
- Thus far, vaccine candidates utilizing mRNA technologies have seen the greatest development. Last week, Moderna began the first human trial for a COVID-19 vaccine, exploring the vaccine’s safety at three different doses. BioNTech, collaborating with Pfizer, expects to enter Phase I trials in late April. Another mRNA candidate produced by CureVac, with funding from CEPI, hopes to start safety trials in June. While showing great promise, these vaccines are still months away from beginning to test efficacy, leaving production of an effective vaccine 12 to 18 months away.
Recent Global Developments
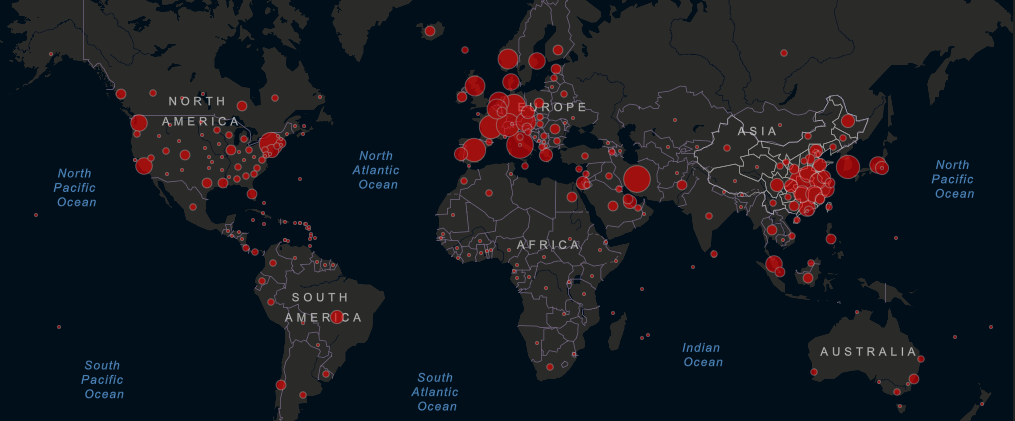
Epidemic spread: One week after the epicenter of the pandemic seemed to shift decisively to Western Europe, centered on Italy, it is shifting again, now focused on New York and the rest of the U.S. Less than three weeks since the first reported case, the infection rate in New York has accelerated sharply. Cases in the state now account for almost half the national total and nearly 7% globally – although these are likely underestimates given U.S. challenges introducing mass testing. Worldwide, as of Thursday, March 26 at 11:00am ET, the Center for Systems Science and Engineering at Johns Hopkins University reported 492,603 confirmed cases and 22,184 deaths attributed to COVID-19.
Populations on lockdown: On Tuesday, March 24, Indian Prime Minister Narendra Modi shut down the nation, confining 1.3 billion people to their homes. The 21-day “total ban of coming out of your homes” is the most aggressive action taken by any country thus far. While such extreme measures could limit the spread of the virus, as was seen in China, many are concerned about the repercussions for the country’s most vulnerable populations. An estimated 50 million Indians live in poverty, making a 21-day work cessation a dangerous proposition. Millions more live in densely populated slums with a lack of clean water and adequate sanitation, limiting their ability to practice social distancing and use effective hand-washing techniques. Concerns about severe economic impact have stymied social-distancing measures in the U.S. As of Thursday, March 26, some form of shelter-in-place order has been enacted in 36 states, restricting movement except for “essential services” – although the definition varies by state, including big-box retailers in some and marijuana dispensaries in others. President Trump has bristled at extensive closings and stated on Tuesday, March 24, “I would love to have the country opened up and just raring to go by Easter,” a move that public health experts advise would be disastrous for the nation’s infection rate and death toll.
Access challenges: As COVID-19 continues to ravage populations around the world, patients and providers are seizing on all possible treatments. Despite lack of evidence, the anti-malarial medicines chloroquine and hydroxychloroquine have been promoted by President Trump as potential treatments for COVID-19. These claims, as yet untested by the FDA, have led to acute shortages of the medication, with people hoarding in the event they become infected – even though improper use can be fatal. At the same time, there have been reports that patients suffering from lupus, rheumatoid arthritis and other conditions who rely on these medications are unable to obtain them.
Other medications that have shown promise, including Gilead’s antiviral remdesivir, have become more difficult to obtain. On Sunday, March 22, Gilead announced it would restrict compassionate use for the compound, citing “overwhelming demand.” The following day, on
Monday, March 23, the FDA granted remdesivir Orphan Drug status for the treatment of COVID- 19, giving Gilead exclusivity for the medication and preventing the FDA from registering a generic version for use against COVID-19 for seven years. After facing harsh criticism, on Wednesday, March 25, Gilead asked the FDA to rescind this special status.
Other pharmaceutical companies, such as AbbVie, are taking steps to remove restrictions to improve access to their medications. On Monday, March 23, AbbVie announced it will relinquish its patents globally on the HIV medication Kaletra (lopinavir/ritonavir), which is being studied as a potential treatment for COVID-19. Patents were set to expire in 2026 in certain countries, and Israel issued a compulsory license for Kaletra prior to the announcement.
From the Experts
“The future of this pandemic, to a greater extent, will be determined by what happens invery large, densely populated countries. So it’s really important that India continues to take aggressive action at the public health level to contain, control, suppress this disease.”
– Dr. Michael Ryan, Executive Director, WHO Health Emergencies Programme
Monday, March 23
“But we’re not prisoners to statistics. We’re not helpless bystanders. We can change the trajectory of this pandemic.”
– Dr. Tedros Adhanom Ghebreyesus, WHO Director-General
Monday, March 23
“The human costs of the coronavirus pandemic are already immeasurable and all countries need to work together to protect people and limit the economic damage…The outlook for global growth is a recession at least as bad as during the [2008] global financial crisis or worse.”
– Kristalina Georgieva, Managing Director, International Monetary Fund
Monday, March 23
“New York is the canary in the coal mine. New York is going first. We have the highest and the fastest rate of infection. What happens to New York is going to wind up happening to California, and Washington state, and Illinois, it’s just a matter of time. We’re just getting there first.”
– Andrew Cuomo, Governor, State of New York
Tuesday, March 24
“If you can’t handle these 21 days, this country will go back 21 years.”
– Narendra Modi, Prime Minister, India
Tuesday, March 24
“The truth is that protecting people and protecting the economy are not mutually exclusive,” DeWine said. “In fact, one depends upon the other. We save our economy by first saving lives. And we have to do it and do it in that order.”
– Mike DeWine, Governor, State of Ohio
Tuesday, March 24
Additional Resources
- WHO COVID-19 Information and Guidance
- WHO Situation Reports, March 23, March 24, March 25
- White House Coronavirus Task Force Press Briefings, March 23, March 24, March 25
- The Lancet COVID-19 Resource Centre
- CDC Coronavirus Resource Page
- Kaiser Family Foundation Coronavirus (COVID-19) Resources
- International Monetary Fund Policy Tracker
- U.S. Chamber of Commerce Foundation Corporate Aid Tracker
- JUST Capital Corporate Response Tracker
- Page Society COVID-19 Toolkit
What We’re Reading
- I fought Ebola. Here is my advice for health workers fighting COVID-19, Adam Levine, STAT News
- Trump wants to ‘reopen America.’ Here’s what happens if we do, Nicholas Kristof and Stuart A. Thompson, The New York Times
- The virus can be stopped, but only with harsh steps, experts say, Donald McNeil, The New York Times
- Remembering America’s global connections in the time of coronavirus, Sten Vermund and Chris Collins, Think Global Health
- “Take as Directed: Coronavirus Crisis Update” Podcast, CSIS