On Wednesday, March 18, Rabin Martin, as the Secretariat for the Private Sector Roundtable for Global Health Security (PSRT), hosted a fourth call in a series on the latest health and economic impacts of COVID-19. Dr. Patrick Osewe, Chief, Health Sector Group, Asian Development Bank, discussed the economic outlook for Asian industry, specifically tourism and manufacturing, and outlined the Bank’s funding strategy given rapid and continued decline of Asian markets. Dr. Osewe emphasized the importance of investments to meet immediate health needs, as well as investments in short-term preparedness to protect countries against further COVID-19 transmission, long-term preparedness for future health emergencies and to support small-to-medium sized enterprises, which are critical to the burgeoning economies of several low- and middle-income countries in Asia. On Wednesday, March 18, the Asian Development Bank announced a $6.5 billion package to provide for the immediate needs of its developing member countries.
Kate Dodson, Vice President, Global Health Strategy, UN Foundation, introduced the COVID-19 Solidarity Response Fund – a new donation platform established by the UN Foundation and the Swiss Philanthropy Foundation to support the WHO and partners in their COVID-19 responses. Learn more about the COVID-19 Solidarity Response Fund below.
PSRT member companies and the Secretariat at Rabin Martin are working in earnest to connect the private sector with key actors to exchange information and meet needs in the COVID-19 response as they arise. For more detailed notes on each call, or to be placed on the invitation list for future calls, please contact [email protected].
Recent Global Developments
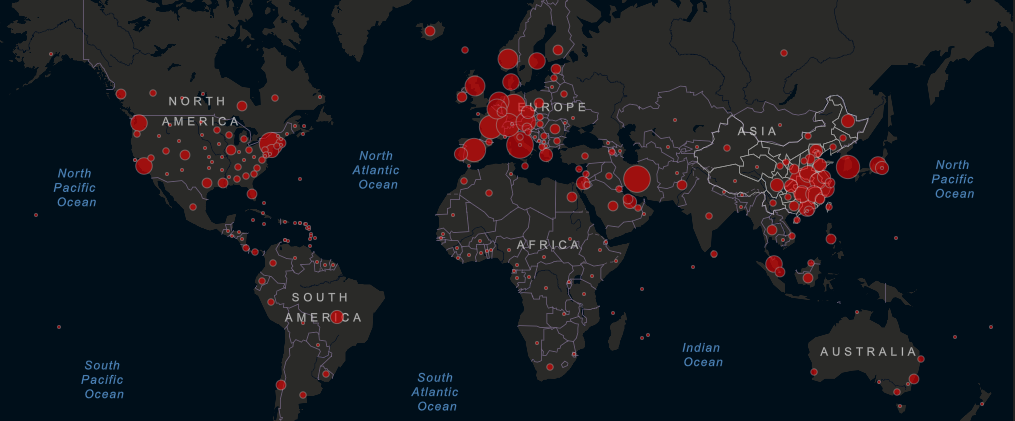
Epidemic spread: As of Thursday, March 19 at 1:00pm ET, Center for Systems Science and Engineering at Johns Hopkins University reported 230,055 confirmed cases and 9,358 deaths attributed to COVID-19. Following aggressive actions to contain the spread of the epidemic, including the introduction of early travel bans and extensive contact-tracing and testing, Asian nations like China, South Korea and Singapore are seeing a slow in new infections, offering vital lessons for other countries. As people China reports an ever growing number of recovered patients, the center of the pandemic has shifted to Western Europe. With 35,713 cases, Italy has the largest epidemic outside of China, leading the Prime Minister Giuseppe Conte to enact a nationwide lockdown on March 9, 2020.
International extreme measures: Restrictive policies on social mobility are now in place in a growing number of as countries around the world to contain the pandemic. In Italy, France and Austria, among others, these include school closures, bans on public gatherings and reduced mass transit service. In the U.S. more than 25 states have closed schools, as of Wednesday, March 18 at 7pm ET, and San Francisco became the first city to introduce a shelter-in-place order, closing all non-essential businesses. Many countries in Western Europe have shut their borders, and by Tuesday, March 17, the EU announced it would close its external borders for 30 days. Adding to last week’s travel ban on foreign nationals from the EU, on Wednesday, March 18, the U.S. announced it would close its border with Canada for non-essential travel.
Funding: The funding required to mitigate the impact of the COVID-19 pandemic is staggering, given wide-ranging needs, from strengthening the supply chain, to establishing and maintaining intensiveness care units, to accelerating vaccine development. The global funding response has been slow in spite of the growing severity of the situation. UK Chancellor Rishi Sunak announced a “whatever it takes” package amounting to 15% of UK GDP – nearly $400 billion total. On Wednesday, March 18 President Trump signed into law a bill to ensure paid leave benefits to many Americans, part of a broader aid package to fight the effects of the pandemic. On Friday, March 13, WHO, the UN Foundation and the Swiss Philanthropy Foundation launched the COVID-19 Solidarity Response Fund. This first-of-its kind fund enables individuals, corporations, foundations and other institutions to contribute directly to global response efforts. Facebook and Google have offered their support to the fund, pledging to match donations raised through their platforms. On Tuesday, March 17, Bloomberg Philanthropies announced the launch of a $40 million Coronavirus Global Response Initiative which will support low- and middle-income countries slow and prevent the spread of COVID-19, in partnership with Vital Strategies. This morning, the Rockefeller Foundation committed $20 million to accelerate short- and long-term pandemic preparedness and response and to address systemic gaps in basic human services.
Notable Industry Developments
WHO Director-General Dr. Tedros Adhanom Ghebreyesus announced the “SOLIDARITY Trial,” an international trial of experimental COVID-19 treatments to ensure robust data to accelerate treatment development.

Takeda Pharmaceuticals foresees a speedy approval for its experimental COVID-19 treatment. The therapy, which is derived from blood plasma of recovered patients, uses the same manufacturing process as Takeda’s other approved immunoglobulin products.
On Monday, March 16, Moderna began testing its experimental vaccine mRNA-1273 in Seattle, the first human trial for a COVID-19 vaccine. The trial examines the vaccine’s safety at three different doses. Later trials will explore the vaccine’s efficacy.
As the U.S. struggles to scale up testing across the country, this week the FDA issued three emergency use authorizations (EUA) for new tests. Hologic, Laboratory Corporation of America (LabCorp), Abbott Laboratories received EUAs for the Panther Fusion SARS-COV-2 Assay, COVID-19 RT-PCR test and RealTime System respectively. FDA reports more than 90 test developers have sought guidance to bring tests through the EUA process since the January.
U.S. Food and Drug Administration and Abbott Laboratories
Johnson & Johnson is evaluating a slate of vaccine and treatment candidates for COVID-19 after announcing there is insufficient evidence to suggest its antiretroviral darunavir is effective in treating COVID-19. In partnership with Beth Israel Deaconess Medical Center, researchers are examining multiple vaccine prospects and aim to identify a candidate by the end of the month and initiate a clinical study by end of the year.
From the Experts
“Hope is not a strategy. We are still very much in the up cycle of this epidemic.”
– Dr. Michael Ryan, Executive Director, WHO Health Emergencies Programme
Thursday, March 12
“You cannot fight a fire blindfolded. And we cannot stop this pandemic if we don’t know who is infected.”
– Dr. Tedros Adhanom Ghebreyesus, WHO Director-General
Monday, March 16
“I want to speak particularly to our largest generation now: our millennials. They are the core group that will stop this virus. They intuitively know how to contact each others without being in large social gatherings.”
– Ambassador Deborah Birx, Coordinator, United States Government Activities to Combat HIV/AIDS, and White House Coronavirus Response Coordinator
Monday, March 16
“We tend to think that we’re not going to be able to mitigate or contain without testing. They complement each other in some respects, but they’re separate channels. Even if we had no testing, we should be doing what we’re doing now.”
– Dr. Anthony Fauci, National Institute of Allergy and Infectious Disease Director
Tuesday, March 17
“We need to democratize and scale the testing system by having a CDC website that people go to and enter their situation.”
– Bill Gates, Co-Founder, Bill & Melinda Gates Foundation
Wednesday, March 18
Additional Resources
-
White House Coronavirus Task Force Briefings, March 16, March 17, March 18
-
Private Sector Roundtable for Global Health Security Business Resource Guide