Representatives from the Department of State yesterday hosted a third call in a series on the latest health and economic impact of COVID-19. Representatives from the U.S. Department of State briefed a cross-industry group of companies, noting that travel guidance has recently changed to Level 3 (Reconsider Travel) and Level 4 (Do Not Travel) for specific regions within Italy and South Korea. The State Department along with the U.S. Centers for Disease Control and Prevention (CDC) and U.S. Department of Health and Human Services (HHS) added that they are in close contact with government counterparts in those countries.
The State Department said it has been pleased with how countries have been transparent in their reporting on the epidemiology of COVID-19 – in alignment with WHO guidelines, the Global Health Security Agenda and International Health Regulations.
PSRT member companies and the Secretariat at Rabin Martin are working in earnest to connect the private sector with key actors to exchange information and meet needs in the COVID-19 response as they arise.
Next week’s call will include Dr. Patrick Osewe, Chief of the Asian Development Bank’s Health Sector Group, to discuss the crippling impact of COVID-19 on Asia’s travel industry. To join the call and learn more about the PSRT, please contact us here.
Recent Global Developments
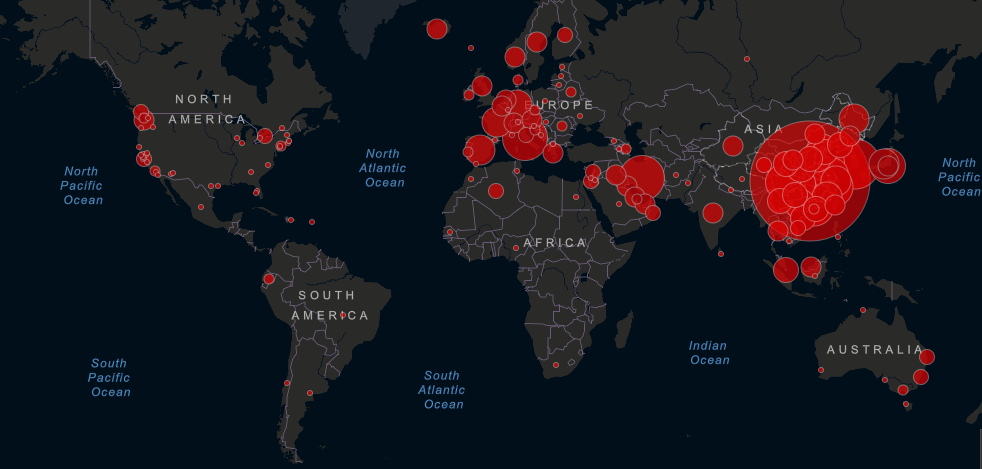
Epidemic spread: As of Thursday, March 5, there were 95,270 confirmed cases and 3,280 deaths attributed to COVID-19, according to WHO. The virus has infected people in at least 81 countries, with the highest density of cases remaining in China, South Korea, Italy, Iran and Japan.
Finance mobilization: On Tuesday, March 3, the World Bank announced it would be making available an initial financing package of up to $12 billion to provide immediate support to countries responding to the health and economic impacts of the epidemic. On Wednesday, March 4, U.S. Congress authorized an $8 billion emergency response package to support the work of CDC and other agencies addressing the outbreak.
Supply shortage: As the number of confirmed and suspected cases of COVID-19 continues to rise, frontline healthcare workers are facing a dangerous shortage of personal protective equipment (PPE), including masks, gowns, goggles and gloves. WHO has responded by shipping nearly 500,000 sets of PPEs to workers in 47 countries, but the global supply cannot continue to support the growing demand.

Notable Industry Developments
Moderna Inc. has shipped the first batch of its COVID-19 vaccine candidate to U.S. government researchers at the National Institute of Allergy and Infectious Diseases (NIAID) for the first clinical trials in humans, slated to begin by the end of April. Results are expected in late summer.
Gilead Sciences announced it is recruiting 1,000 patients for Phase 3 clinical trials for its investigational compound remdesivir. These studies will take place at medical centers in countries with highest number of diagnosed cases.
Sanofi Pasteur is partnering with the U.S. Biomedical Advanced Research and Development Authority, expecting to have a viable vaccine candidate to test in the lab within six months and in people within 12-18 months. Sanofi will build on its previous work developing a vaccine against SARS.
CureVac and the Coalition for Epidemic Preparedness Innovations (CEPI) announced a public-private partnership to accelerate development of a potential vaccine using mRNA, with CEPI providing up to $8.3 million in additional funding to accelerate vaccine development, manufacturing and testing.
From the Experts
“At this pivotal moment, every effort must be made to push back against the outbreak. These crucial funds will support our global efforts to bolster weaker health systems and inform children, pregnant women and families about how to protect themselves.”
– Henrietta Fore, UNICEF Executive Director
Sunday, March 1
“I want to caution everybody… the whole [vaccine development] process is going to take a year, a year and a half at least.”
– Dr. Anthony Fauci, NIAID Director
Tuesday, March 3
“We can’t stop COVID-19 without protecting our health workers.”
– Dr. Tedros Adhanom Ghebreyesus, WHO Director General
Tuesday, March 3
“We should be cautious and take appropriate measures to prepare and protect ourselves, but we should not be afraid.”
– Vice Admiral Jerome Adams, Surgeon General of the United States
Tuesday, March 3
“We need to slow the spread of disease to the point where our health care system can continue to handle the load.”
– Patty Hayes, Director of Public Health, Seattle & King County
Thursday, March 5