On Wednesday, May 6, Rabin Martin, as the Secretariat for the Private Sector Roundtable for Global Health Security (PSRT), hosted an eleventh call in a series on the latest health impacts of COVID-19. The call featured Dr. Sandro Galea, Dean and Robert A. Knox Professor, School of Public Health, Boston University.

Dr. Galea discussed how the social determinants of health are relevant to high-income countries as well as low- and middle-income ones and are especially important to consider in the global response to COVID-19.
Beginning with an overview of social determinants, he used the example of mapping diabetes incidence in Boston to the city’s public transportation network to show how social determinants influence population health outcomes. The data show significant disparities in diabetes between communities that are only a mile or two apart. These differences are particularly striking in a city with the highest per capita population of doctors in the United States. Although all residents live near world-class hospitals, the incidence of diabetes is twice as high in the southern vs. the northern part of the city, which correlates with the disparities in income and education levels in these communities.
Dr. Galea’s overarching message was that many aspects of health are driven by where you live, what you eat, where you play, your type of work and wages. It is critically important to “understand how the world around us shapes our health.” Social determinants are the “ubiquitous factors” that we don’t see and, consequently, don’t pay attention to when we talk about health. These include quality of housing, gender equity, air quality, livable wages, presence/absence of violence and availability of affordable nutritious food, among others. Instead we often focus on behavior change even though “the efforts to encourage healthy eating are futile if we don’t pay attention to higher-level upstream determinants.”
He outlined four of the most important social determinants that are driving health globally and are connected directly to COVID-19:
- Urbanization – the majority of the world now lives in urban areas and the built environment affects exercise, quality of education, risk of infection, congregation and more – yet this is something we create and control
- Population aging – more people are over 65 than under 5 for the first time in history. “We cannot think about COVID-19 without recognizing that it’s a disease among people who are older – both in high- and low-income countries – and population aging is why we haven’t seen an explosion of cases in lower- and middle-income countries (LMICs)”
- Migration – the enormous number of people living in unstable conditions makes it difficult to put structures in place to mitigate disease; notably, the recurrent COVID-19 cases in Singapore were all in migrant communities that had not been on the government’s radar
- Climate change – which will shape health for the next 30-40 years, especially deteriorating air quality and conditions that lead to large-scale disasters
In addition to these determinants, LMICs are also worse off compared to high-income countries because of more fragile health systems, which are less resilient and adaptable, and vast economic inequalities. For people barely subsisting, it is “impossible to withstand the stressor of a pandemic or a lockdown and its consequences.” Dr. Galea added that, because of the differences in social determinants, the western approach to lockdown is not applicable to other regions.
In terms of recommendations for the COVID-19 response, Dr. Galea argued that the solution is to stratify risk and to target resources (especially Office of Development Assistance funding) toward testing, contact tracing and strategic isolation of people at high risk – adding that mass physical distancing is unrealistic in LMICS. He called this the right approach for both LMICs and high-income countries and “the only supportable approach in LMICS.” He urged against a “one size fits all blanket shutdown of economies and societies,” because it will only mitigate the virus’ transmission in the short term, but will not work in the long term, noting that economic damages will haunt these countries longer than COVID-19.
He concluded that we need to understand rapidly who is at risk and focus attention on those populations with strategies built on risk stratification. He predicted that the path forward will require grappling with civil liberties issues and employer expectations of being present at work. The challenge before us is how to balance risks and make uncomfortable decisions – and to do so very quickly.
Pandemic Spread
Even as COVID-19 cases in the U.S. climbed past 1.1 million, on Tuesday, May 5, the Trump administration suggested it was time to dismantle the White House Coronavirus Task Force as the country shifts its focus to economic recovery. However on Wednesday, May 6, the administration changed course after receiving criticism of the proposal, with experts calling it premature, and has since stated that the Task Force will continue for the duration of the crisis and will expand its membership.
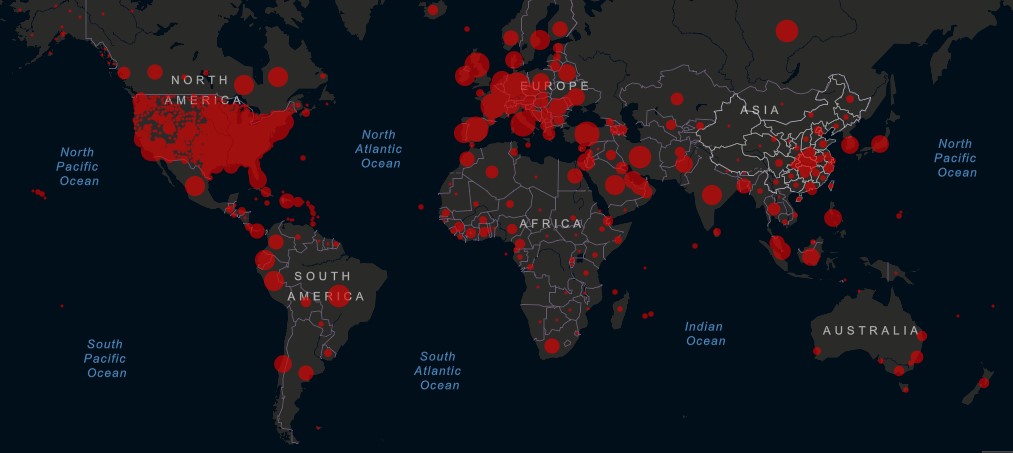
In just over four months since the first reported case of COVID-19 in China, the world is approaching 4 million cases. The U.S. remains the most active epidemic hotspot, suggesting the pandemic is far from over. With 1,229,089 cases and 73,435 deaths, the U.S. accounts for a third of global burden.
States hit hard early in the crisis, such as New York, are experiencing a decline in new cases due to the extensive measures put in place to flatten the curve. Concerningly, incidence is increasing elsewhere in the U.S. and hotspots are arising in new cities such as Chicago and Los Angeles and in rural areas such as Nebraska. The continued rise in cases, cresting this week at 25,500 a day, has left experts troubled by mixed messaging by the Trump administration and the concurrent state-level initiatives to phase out social distancing regulations.
Worldwide, as of Thursday, May 7 at 9:30 am ET, the Center for Systems Science and Engineering at Johns Hopkins University reported 3,778,179 confirmed cases and 264,437 deaths attributed to COVID-19.
Industry Developments
“Never in modern times have such high hopes for millions of lives rested on one single company.”
On Friday, May 1, the U.S. FDA announced an emergency use authorization for Gilead’s experimental compound, remdesivir, for the treatment of COVID-19. While clinical studies to determine efficacy continue, the drug was shown to shorten the time to recovery in some patients. Potential production issues are already arising, with concerns about the company’s ability to develop and distribute sufficient amount of product to satisfy global demand. Gilead has pledged to donate doses for an estimated 140,000 patients, with the aim of supplying affordable treatment to over a million patients by the end of 2020. Gilead CEO Daniel O’Day noted their commitment to building a global manufacturing network, using technology transfer and licensing, to ensure a robust global supply chain scaled to meet anticipated need.
One explanation for the U.S.’s slow introduction of testing at the outset of the pandemic has focused on the onerous regulations required for FDA approval – though the delays caused by inefficiencies in Federal efforts to coordinate production and distribution of tests and supplies cannot be discounted. In an effort to course correct as it began reviewing antibody tests, the FDA published wide, flexible guidelines that allowed for a proliferation of unverified tests to enter the market. An evaluation of 14 antibody tests in late April spurred criticism that the agency’s leniency had allowed faulty products to reach consumers. On Monday, May 4, the FDA backtracked and released new stricter policy guidelines for antibody tests: companies must submit applications for emergency use authorizations within 10 days of the policy change or withdraw their product from the market.
- On Thursday, April 30, AstraZeneca entered a partnership with the Jenner Institute to manufacture and distribute the Institute’s vaccine candidate, currently known as ChAdOx1 nCoV-19. Phase I clinical trials of the vaccine began last week in Southern England; data are expected next month.
- Lilly is doubling down on efforts to develop antibodies to combat COVID-19. On Monday, May 4, Lilly purchased rights to co-develop antibodies with China’s Junshi Biosciences for $10 million upfront. This collaboration is on top of Lilly’s existing partnership with AbCellera announced in March.
- During its Q1 earnings call on Tuesday, May 5, Regeneron announced it is beginning large-scale manufacturing in anticipation of starting clinical trials of its artificial antibody cocktail in June. Pending favorable results, the antibody cocktail could be available for commercial use in the fall.
- After launching trials in Germany late last month, Pfizer and BioNTech initiated the U.S. Phase I/II clinical trials of four mRNA vaccine candidates on Tuesday, May 5. Preparing for positive results, Pfizer is planning to invest at risk to scale up vaccine production to generate millions of doses by the end of 2020.
- On Tuesday, May 5, Sanofi announced it plans to enroll thousands of people for trials of the experimental vaccine it is developing in partnership with GlaxoSmithKline. Early stage trials are set to begin in September, but the companies hope the large number of subjects will expedite the process and yield important data.
From the Experts
“Comprehensive, coordinated public health measures are critical to slow transmission and to save lives. But even countries that have taken such steps remain in jeopardy. And the virus is still likely to strike many countries that are least able to cope. In an interconnected world, none of us is safe until all of us are safe.”
António Guterres, Secretary-General, United Nations
Monday, May 4
“In the space of just a few hours we have collectively pledged 7.4 billion euros ($8.1 billion) for vaccine, diagnostics and treatment. This will help kick-start unprecedented global cooperation.”
Ursula von der Leyen, President, European Commission
Monday, May 4
“How many deaths and how much suffering are you willing to accept to get back to what you want to be, some form of normality, sooner rather than later?”
Anthony Fauci, Director, NIAID
Monday, May 4
“The fundamental question we’re not articulating is how much is a human life worth?”
Andrew Cuomo, Governor, State of New York
Tuesday, May 5
“We need to increase our preparedness for the future. And we need to fight inequality and put rights and people at the center of the response.”
Winnie Byanyima, Executive Director, UNAIDS
Wednesday, May 6
“Let us be clear: without a corona vaccine we will never be able to live normally again. The only real exit strategy from this crisis is a vaccine that can be rolled out worldwide. Despite all our efforts, it is still not certain that it will be possible to develop a vaccine against the coronavirus. In the worst case, we will be able to do nothing but try to limit the damage.”
Peter Piot, Director, London School of Hygiene & Tropical Medicine
Wednesday, May 6
Additional Resources
Reports from International Governments and Bodies
- WHO COVID-19 Information and Guidance
- WHO Situation Reports, May 4, May 5, May 6
- CDC Coronavirus Resource Page
- COVID-19 Health Systems Response Monitor
- NCD Alliance COVID resources relevant to NCDs
Funding and Policy Trackers
- International Monetary Fund Policy Tracker
- Kaiser Family Foundation Coronavirus Policy Tracker
- U.S. Chamber of Commerce Foundation Corporate Aid Tracker
- Devex Interactive Funding Tracker
Resource Pages and Market Research Literature
- JAMA Resource Center
- The Lancet COVID-19 Resource Centre
- 2019 Novel Coronavirus Research Compendium (NCRC)
- WIPO COVID-19 IP Policy Tracker
- The COVID Tracking Project
- PharmaIntelligence: Coronavirus – What will the Impact Be?
- Health Affairs Resource Center
- STAT Preparedness Tool
- International Association of National Public Health Institutes COVID-19 Resources
- Primary Health Care Performance Initiative Forum
- U.S. Global Leadership Coalition COVID-19 Issue Briefs
- Prevent Epidemics Weekly Science Review
Communications Toolkits
What We’re Reading
Profits and Pride at Stake, the Race for a Vaccine Intensifies – David E. Sanger, David D. Kirkpatrick, Carl Zimmer, Katie Thomas and Sui-Lee Wee; The New York Times
In the Race for a Coronavirus Vaccine, We Must Go Big. Really, Really Big. – Susan Athey, Michael Kremer, Christopher Snyder and Alex Tabarrok; The New York Times
How A.I. Steered Doctors Toward a Possible Coronavirus Treatment – Cade Metz; The New York Times
Efforts to Beat Back the Coronavirus are Critical. They’re Also Making Clinical Trials Harder – Andrew Joseph, STAT News
Will poor countries get the coronavirus treatments they need? – Andrew Jack, Financial Times
Nations Back Push for Universal Access to COVID-19 Vaccines – James Paton, Bloomberg
Should Covid-19 Investors Leave Money on the Table? – Suerie Moon and Nadya Wells, Barron’s
ICT COVID-19 Response: Partnering with Technology Companies to Combat COVID-19 – World Economic Forum